Talking Genomics – Understanding The Random Shuffle of Genes


Genomic testing is widely considered the most significant transformational technology in livestock breeding since the introduction of Estimated Breeding Values, or EBVs, with genomic information now routinely incorporated into beef, sheep and dairy genetic evaluations around the globe.
To understand the value of genomic testing for the prediction of breeding value in these genetic evaluations, one must first understand how genes are inherited from one generation to the next.
An animal’s genes are contained within its DNA, which is bundled into chromosomes. Livestock have pairs of chromosomes, with the number of chromosome pairs in an animal’s DNA varying across the different species (table 1). For example, cattle have 30 pairs of chromosomes, or a total of 60 chromosomes. One chromosome in each chromosome pair is inherited from the animal’s sire, and one from the animal’s dam.
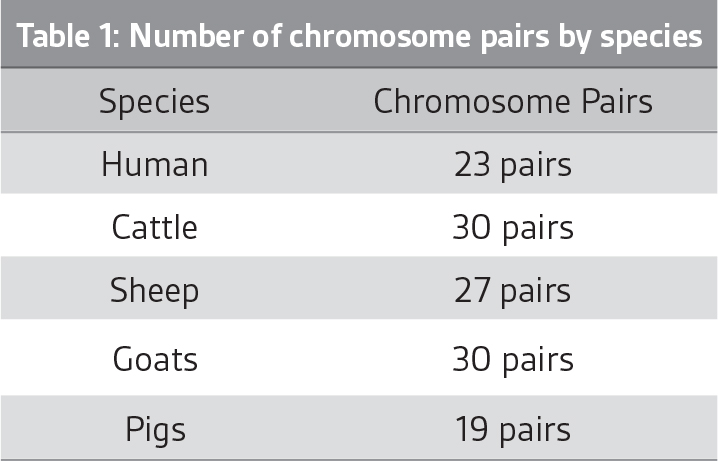
When the animal reproduces, for example, a sperm is produced by a sire, one of the chromosomes in each pair is selected at random and passed on to the progeny. So, when a sperm is produced, the selection of chromosomes that are passed on is similar to flipping a coin numerous times (i.e. 30 times in cattle, 27 times in sheep), with a different combination of heads and tails returned each time a new sperm is produced.
If we label the chromosomes the sire inherited from his father as blue (paternal) and the chromosomes inherited from his mother as pink (maternal), the number of possible combinations of the sire’s paternal and maternal chromosomes that can be passed onto the progeny is illustrated in Figure 1.
Delving into this in more detail, the number of possible chromosome combinations that can be passed on in cattle is 1,073,741,824 (i.e. 230), while in sheep the number of possible chromosome combinations is 134,217,728 (i.e 227). And this number ignores the exchange of segments between paternal and maternal chromosomes that occurs in a biological process called recombination.
The manner in which chromosomes are passed from one generation to the next is often described as the progeny receiving a random sample of the sire’s genes, or mendelian sampling, and with the number of possible combinations, the genes passed on in no two sperm are exactly alike. The same number of possible combinations is true for each egg produced by a dam, meaning the number of possible chromosome combinations inherited by animals from their parents is literally in the billions.
Think for a moment about your favourite set of full siblings, being brothers or sisters with the same parents.
Perhaps this is your favourite set of embryo flush mates, your brothers and sisters, or your children. The dissimilarity between full siblings may be striking, for example, one may be short and the other tall, one may have light hair and the other dark hair, or one may be laid-back and the other excitable.
The similarities and dissimilarities between full siblings are due to differences in the chromosomes that they have inherited, and the environment they have experienced.
So what does this mean for breeding values?
In their simplicist form, the estimated breeding value (EBV) calculated for an animal can be thought of as one half of the sire’s breeding value, plus one half of the dam’s breeding value, plus the mendelian sampling term. The mendelian sampling term represents the animal’s difference from the average of its parents’ breeding values and is due to the random sample of genes and chromosomes that the calf inherited.
EBVCALF = ½ EBVSIRE + ½ EBVDAM + mendelian sampling term
In a traditional, pedigree based genetic evaluation, there is no data available when an animal is born, so the Mendelian sampling term can not be estimated, and the animal’s EBV is reported as the parent average. This is often referred to as the ‘mid-parent’ EBV value.
As performance data is subsequently collected on the animal, and the animal’s progeny, the mendelian sampling term can be estimated, and the animal’s EBV either increases, decreases or remains unchanged, reflecting the random sample of genes that the animal has inherited from its parents.
But this approach has limitations and it can be problematic collecting effective performance information on the animal to enable the mendelian sampling term to be estimated. For example, traits that are difficult or expensive to measure, traits that can’t be measured prior to the animal entering the breeding herd, traits that have a low heritability, or animals that are in small contemporary groups.
This is where genomics now provides considerable value.
In a genetic evaluation that incorporates genomic information, such as the industry single step genetic evaluations that are conducted in the beef and sheep industries in Australia and New Zealand (e.g. BREEDPLAN, Sheep Genetics), predominantly for seedstock animals, genomic data provides an insight into the random sample of chromosomes that an animal has inherited, which enables an estimate to be made of the Mendelian sampling term early in an animal’s life.
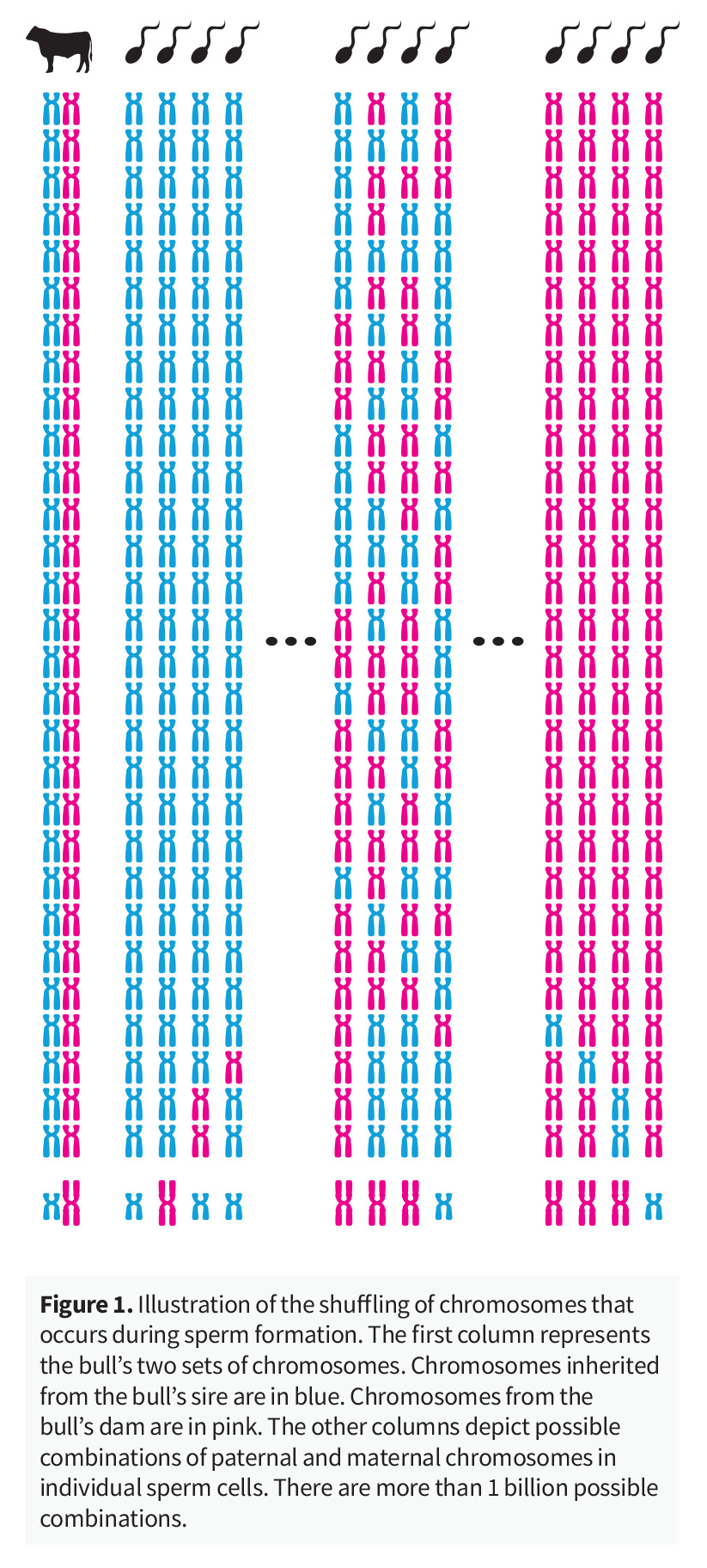
For example, in the real world example provided in Figure 2, based on averages, the animal would be expected to share 25 percent of its genes with each of its grandparents. But, due to the random shuffle of genes and chromosomes, this percent can vary greatly. Genomic testing reveals that the animal shares 25.8 percent of their genes with their paternal grandsire (orange chromosome pair), and 15.4 percent of their genes with their maternal grandsire (green chromosome pair).
Knowledge of the chromosomes that an animal has inherited via genomic testing, and the resultant ability to better estimate the mendelian sampling term, results in the availability of more accurate and reliable EBVs for animals.
To further discuss the development of a genomic testing program for your livestock breeding program, contact staff at Neogen Australasia.
Reference: ‘The Random Shuffle of Genes: Putting the E in EPD’
Andrew Byrne, Senior Technical Product Specialist, Neogen Australasia. From our partner, Neogen.